CD4-positive T helper cells orchestrate the cellular and humoral immune response to various pathogens, including viruses, bacteria, fungi, and parasites. Different subsets of T helper cells have been characterized based on their functional properties. Among these, T follicular helper (Tfh) cells provide help to B cells for efficient antibody production. In contrast, dysregulated Tfh cells can also cause autoimmunity and allergies. Interestingly, depending on the context, Tfh cell frequencies have been correlated with either positive or negative outcomes for various tumor entities. Recently, Tfh cells have emerged as central intermediaries of other T helper cell-mediated immune responses that involve the generation of certain effector and memory T helper cell populations. Nevertheless, the precise relationship between Tfh and other T helper cell subsets remains unknown.
We are combining cellular and molecular immunology techniques and state-of-the-art genomic approaches to answer fundamental questions about T helper cell differentiation and plasticity and their impact on adaptive immune responses. It is anticipated that a better understanding of how Tfh cells are regulated on the molecular level and how Tfh cells contribute to T helper cell fate decisions will yield important insights into the rational design of drugs and therapies that target Tfh cells in autoimmune diseases and allergies or boost their function during infection and vaccination.
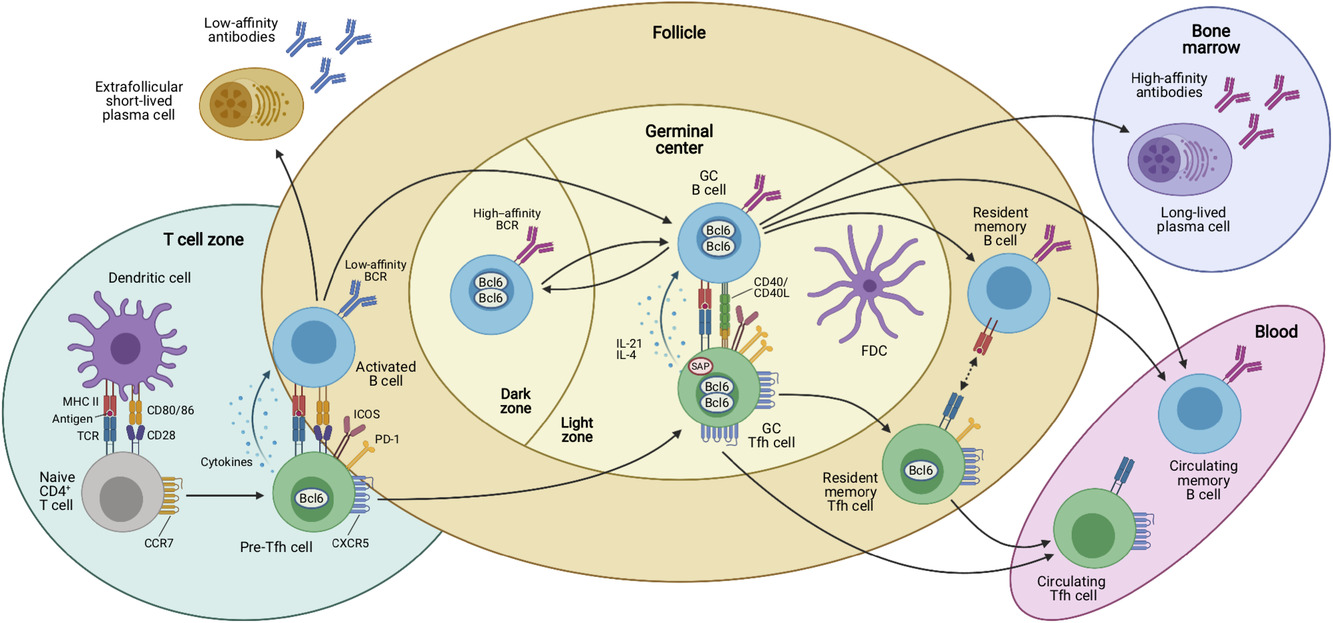
Antigen-dependent multistep differentiation of T follicular helper cells. Naïve CD4+ T cells are primed by dendritic cells (DCs) in the T-cell zones of secondary lymphoid organs such as the spleen or lymph nodes. This antigen-specific interaction is mediated by presentation of processed peptides by MHC-II molecules that are recognized by the cognate TCR. Together with the expression of costimulatory molecules and production of cytokines, these signals induce the Tfh cell differentiation program that is characterized by upregulation of the chemokine receptor CXCR5 and downregulation of CCR7, which allows these cells to migrate to the T/B border where they interact with activated B cells. This second interaction with an APC type coincides with the expression of characteristic costimulatory (e.g. ICOS) and coinhibitory receptors (e.g. PD-1) by the Tfh cells, which may be called “pre-Tfh” cells at this stage. After this cellular interaction, some activated B cells become extrafollicular short-lived plasma cells (PCs) that secrete low-affinity antibodies and some of the interacting T and B cells relocate to the follicle to form germinal centers (GCs). In these highly specialized microanatomical structures that consist of dark zones (DZ) and light zones (LZ), GC Tfh and B cells continue to interact in an antigen-specific manner. GC Tfh cells are more polarized and express higher levels of PD-1, Bcl6, and CXCR5 than “pre-Tfh” cells. In addition, they express the adaptor molecule SAP that is important for interactions with GC B cells and they produce IL-21 and IL-4 that act on the B cells. While GC B cells mutate their immunoglobulin genes and proliferate in the DZ, GC Tfh cells provide essential signals to B cells in the LZ of the GC, where they cooperate with follicular dendritic cells (FDCs) in the selection of high-affinity B-cell clones and in the generation of memory B cells, which recirculate in the blood, and long-lived plasma cells that find their niche in the bone marrow to maintain serological memory through the secretion of high-affinity antibodies. Once the GC reaction is resolved, some memory CXCR5+ CD4+ T cells reside in close proximity with memory B cells in lymphoid organs, while others are released from the draining lymphoid organs and circulate in the blood. Both memory Tfh cell subsets express decreasing amounts of Bcl6, CXCR5, and other costimulatory/inhibitory molecules as compared to their effector counterparts. Upon antigen rechallenge, these cells rapidly acquire the effector functions of Tfh cells and strongly support secondary immune response. Created with BioRender.com. From: Baumjohann & Fazilleau, Eur J Immunol 2021; CC BY-NC-ND 4.0.
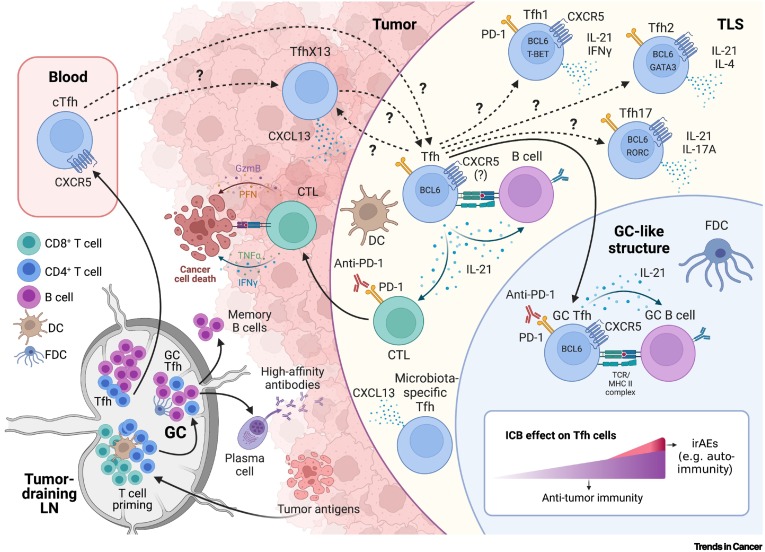
T follicular helper (Tfh) cells in cancer. Different subpopulations of Tfh and Tfh-like cells participate in the antitumor immune response and are affected by immune checkpoint blockade (ICB) therapy. Although some cellular and molecular mechanisms have been elucidated (unbroken arrows), the ontogeny of these cell subpopulations in tumors and tertiary lymphoid structures (TLS) remains largely unknown (broken arrows with question marks). (Bottom left) After priming by dendritic cells (DCs) in tumor-draining lymph nodes (dLNs), activated CD4+ and CD8+ T cells differentiate into effector T cells such as type 1/2/17 T helper (Th1/Th2/Th17) cells and cytotoxic T lymphocytes (CTLs), respectively, which then leave the dLN and migrate to peripheral tissues such as the tumor. Activated CD4+ T cells differentiate into early Tfh cells that interact with B cells to initiate the early extrafollicular antibody response. Some of these Tfh cells leave the dLN to become circulating Tfh (cTfh) cells, and others join antigen-specific B cells and enter the follicle to form germinal centers (GCs). In these microanatomical structures, high-affinity antibodies, long-lived plasma cells, and memory B cells are formed. GCs also harbor follicular dendritic cells (FDCs) that present native antigen to B cells. (Top right) Interestingly, similar GC-like structures consisting of B cells, FDCs, and Tfh-like cells can be found in TLS that form adjacent to or within tumor tissues. TLS-resident Tfh cells may be differentially polarized depending on the environmental context of the tumor to Tfh1, Tfh2, or Tfh17 cell types. IL-21 produced by Tfh cells supports B cells but also CTL function. Other Tfh-like cells are TfhX13 cells and microbiota-specific Tfh cells that produce CXCL13 but lack CXCR5 expression and may contribute to TLS formation. Upon ICB with anti-PD-1, the function of Tfh cells is boosted but may also contribute to the development of immune-related adverse events (irAEs). The precise kinetics and dynamics of this phenomenon are still unknown, but current evidence indicates a positive correlation between antitumor activity and the development of irAEs (bottom right). Created with BioRender.com. From: Gutiérrez-Melo & Baumjohann, Trends Cancer 2023; CC BY 4.0.
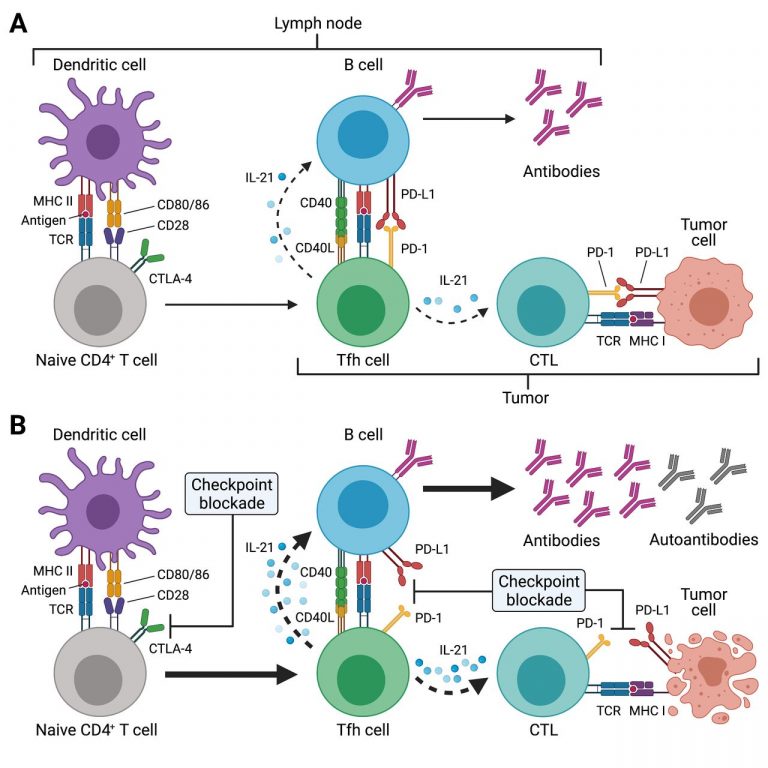
Potential effects of immune checkpoint inhibitor (ICI) treatment on T follicular helper (Tfh) cell responses. (A) Tfh cell differentiation is initiated in the T cell zone of secondary lymphoid organs (SLOs) such as lymph nodes (LNs) by priming of naïve CD4+ T cells through dendritic cells (DCs). This involves presentation of antigenic peptides (eg, derived from drained tumor tissues) on major histocompatibility complex class II (MHC II) and co-stimulation through CD28, which is expressed on T cells. CTLA-4 inhibits CD28-induced proliferation and acts as a break. SLO-resident Tfh cells and tumor-resident Tfh-like cells (as indicated by the different brackets) provide critical help to B cells for antibody responses through T-cell receptor (TCR) recognition of cognate (tumor) antigens presented on MHC II. Tfh and Tfh-like cells deliver co-stimulatory (eg, CD40L) and receive co-inhibitory (eg, PD-1) signals to/from B cells and together with cytokines such as interleukin-21 (IL-21) instruct antibody diversification and affinity maturation. In the context of tumors, IL-21 may also promote the antitumor activity of cytotoxic T cells (CTLs). (B) ICI treatment acts at different stages of the Tfh cell response. Anti-CTLA-4 treatment boosts naïve CD4+ (and CD8+) T cell priming, thus resulting in highly activated and proliferating CD4+ T cells such as Tfh cells (as well as CTLs). Tfh and Tfh-like cells express high levels of PD-1 and blockade of this pathway during ICI treatment may unleash the antibody response, which may also result in the production of autoreactive antibodies. Besides its direct effect on CTLs, PD-1/PD-L1 blockade may also boost IL-21 provision to CTLs by Tfh and Tfh-like cells in LNs and tumor tissues, with the resulting exaggerated CTL response, potentially also driving autoimmune manifestations of immune-related adverse events (irAEs). Created with BioRender.com. From: Baumjohann & Brossart, J Immunother Cancer 2021; CC BY 4.0.
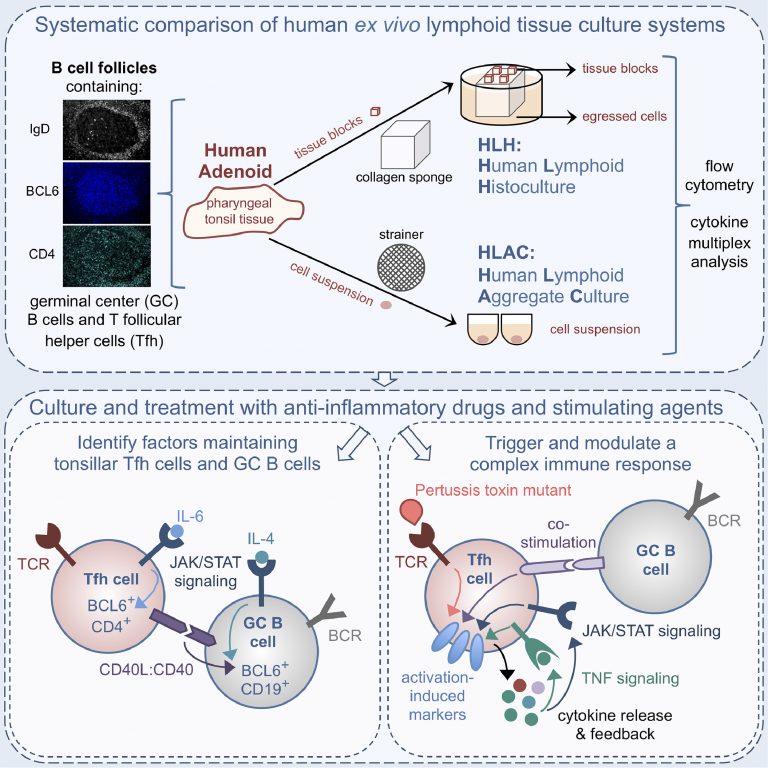
Human tonsil/adenoid tissue-based ex vivo immune organoid cultures: Human lymphoid histoculture (HLH) and human lymphoid aggregate culture (HLAC). The upper panel shows a schematic view of the adenoid culture systems employed to study lymphocytes in human secondary lymphoid tissue ex vivo. Lower left: JAK/STAT signaling contributes to BCL6 maintenance in Tfh and GC B cells, with a role for IL-4 signaling and CD40L in B cells as opposed to IL6R signaling in T cells. Lower right: An optimized activation-induced marker (AIM) assay triggers a complex T cell immune response using enzyme-dead PT protein plus B cell costimulation, leading to upregulation of the AIMs OX40, PD-L1 and CD25 along with cytokine release. Released TNF as well as JAK-dependent cytokine signals contribute to bystander activation and amplification of the response. From: Schmidt et al., eBioMedicine 2020; CC BY-NC-ND 4.0.

Human tonsil/adenoid-derived immune organoids and other complex tissue culturing techniques. (A) Due to their constant antigenic encounters, lymph nodes and other secondary lymphoid organs (SLOs) frequently harbor prominent germinal centers (GCs), which reside in the B cell follicles and are surrounded by the mantle zone that mainly contains naive B cells. GCs are micro-anatomical structures subdivided into dark (DZ) and light zones (LZ). In DZs, B cells proliferate as centroblasts and undergo somatic hypermutation to mutate their antibody receptors. In LZs, B cells (centrocytes) are interacting with follicular dendritic cells (FDCs) and T follicular helper (Tfh) cells, which contribute to selecting higher-affinity B cell clones and determining the fate of these cells to becoming either long-lived antibody-secreting plasma cells or memory B cells. Those B cells that are not selected into these cell fates or that do not re-enter the DZ become apoptotic and die of programmed cell death. (B) As part of the Waldeyer’s ring, palatine tonsils are situated bilaterally in the oral cavity and the pharynx. Nasopharyngeal tonsils (adenoids) are situated in the pharynx more nasally. Together with tubal and lingual tonsils, these SLOs represent the first line of defense of the immune system against oral and airborne pathogens. (C) Often, tonsils and adenoids become too large so that they may obstruct the breathing abilities of an individual and then require (partial) surgical resection. This material, which is mostly discarded after surgery, contains highly activated lymphocytes and GCs and can be used for setting up complex ex vivo immune organoid cultures for research purposes, including human lymphoid histoculture (HLH) and human lymphoid aggregate culture (HLAC). (D) Tonsil-based immune organoid cultures represent versatile experimental systems that can be used to investigate the effects of antibodies, drugs, cytokines, and pathogens/antigens on various immune cell types, including T and B cells. These cultures can also be combined with genetic engineering, such as CRISPR, to dissect molecular processes. (E) Besides tonsil-based immune organoids, other technologies have been developed to mimic SLOs in vitro, including PBMC or lymph node-based organ-on-a-chip approaches. Furthermore, efforts are being made to combine classical organoid cultures, especially from various cancer tissues, with defined immune cell populations to mimic anti-cancer immune responses. Created with BioRender.com/h43i527. From: Moll & Baumjohann, EMBO Mol Med 2025; CC BY 4.0.